Background: Nodular lymphocyte predominant Hodgkin lymphoma (nLPHL) has different histology, clinical presentation, and outcomes than classical Hodgkin Lymphoma (cHL). Positron Emission Tomography (PET) plays an important role in staging and response assessment in cHL, but little is published about the use of PET to guide treatment in nLPHL. A better understanding of the clinical significance of PET response in nLPHL is critical for incorporating PET into prospective trials.
Methods: Children's Oncology Group study AHOD03P1 (NCT00107198) enrolled patients with low-risk nLPHL and AHOD0031 (NCT00025259) enrolled patients with intermediate-risk cHL and nLPHL <22 years of age. AHOD03P1 patients with stage IA and >1 lymph node or stage IIA nLPHL were treated with 3 cycles of doxorubicin, vincristine, prednisone, and cyclophosphamide (AV-PC) chemotherapy, given every 21 days. Patients with a complete response (CR) based on anatomic response and qualitative reading (<mediastinal blood pool) of negative PET on central review did not receive radiation therapy while patients with <CR received 21-Gy involved field radiation therapy (IFRT). AHOD0031 patients with stages IB, IAE, IIB, IIAE, IIIA, IVA with or without bulk disease, and IA or IIA with bulk disease received 2 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) followed by response evaluation. Slow early responders were randomized to an additional 2 cycles of ABVE-PC with or without 2 cycles of dexamethasone, etoposide, cisplatin and cytarabine (DECA) and all received IFRT. Rapid early responders (RERs) received 2 additional cycles of ABVE-PC. RERs with CR were randomized to IFRT or no further therapy. RER/non-CRs were non-randomly assigned to IFRT.
PET scans from both trials available in the Imaging and Radiation Oncology Core (IROC) were retrospectively reviewed and assigned a score of 1-5 on the Deauville 5-point scale (5PS). Patients who did not have evaluable imaging due to either scans not performed, on CD-ROM and inaccessible, or poor quality, were excluded from this analysis. Three-year event-free survival (EFS) was analyzed by 5PS at end of therapy using the Kaplan Meier method.
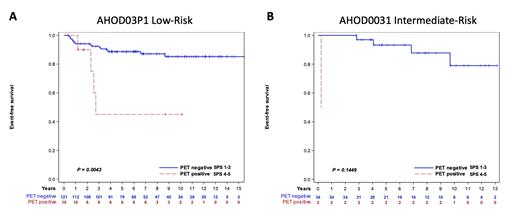
Results: AHOD03P1 enrolled 135 patients with low-risk nLPHL who received chemotherapy and AHOD0031 enrolled 96 patients with intermediate-risk nLPHL. PET imaging available for review at diagnosis in 99 low-risk patients, 92% 5PS=5, and 55 intermediate-risk patients, 98% 5PS=5. Median age 13.4 years (range 16.5), 84.9% male, 29.9% Stage I, 49.8% Stage II, 18.6% Stage III, and 1.7% Stage IV. After 3 cycles of chemotherapy, 106 low-risk patients had available PET scans, 5PS 1 n=50, 5PS 2 n=35, 5PS 3 n=11, 5PS 4 n=7, and 5PS 5 n=3. Three-year EFS for patients with end of chemotherapy 5PS was: 5PS 1 89.9% (95% CI 77.3-95.7%), 5PS 2 97.1% (95% CI 81.4-99.6%), 5PS 3 90.9% (95% CI 50.8-98.7%), 5PS 4 28.6% (95% CI 1.0-71.1%), 5PS 5 66.7% (95% CI 5.4-94.5%) p=0.0081. When categorized as PET negative (5PS 1-3) and PET positive (5PS 4-5), 3-year EFS was 92.6% (95% CI 85.2-96.4%) for PET negative and 45.0% (95% CI 10.6-75.3%) for PET positive patients, p=0.0036 (Figure 1A).
Forty-nine intermediate-risk patients had available PET scans after 2 cycles of chemotherapy, 5PS 1 n=9, 5PS 2 n=26, 5PS 3 n=10, 5PS 4 n=3, and 5PS 5 n=1. Three-year EFS by interim response was: 5PS 1 88.9% (95% CI 43.3-98.4%), 5PS 2 96.0% (95% CI 74.8-99.4%), 5PS 3 100%, 5PS 4 100%, 5PS 5 0%, p<0.0001. Thirty-six patients had available PET scans after 4 cycles of chemotherapy, 5PS 1 n=20, 5PS 2 n=11, 5PS 3 n=3, 5PS 4 n=1, and 5PS 5 n=1. Three-year EFS by response after 4 cycles of chemotherapy: 5PS 1 95.0% (95% CI 69.5-99.3%), 5PS 2 100%, 5PS 3 100%, 5PS 4 100%, 5PS 5 0% p<0.0001. When divided into PET negative (5PS 1-3) and PET positive (5PS 4-5), 3-year EFS 97.0% (95% CI 80.4-99.6%) for PET negative and 50.0% (95% CI 0.6-91.0%) for PET positive patients after 4 cycles of chemotherapy, p=0.1449 (Figure 1B).
Conclusions: At diagnosis, low and intermediate-risk nLPHL lesions are almost always PET avid with 5PS=5. PET response following 3 cycles of AV-PC chemotherapy is highly predictive of outcome in pediatric patients with low-risk nLPHL when considering 5PS=3 as PET negative. Intermediate-risk patients with negative PET following ABVE-PC achieved excellent outcomes. Whether PET maintains prognostic significance in patients with nLPHL treated with rituximab needs to be studied.
Disclosures
Kelly:Seagen: Other: Scientific Steering Committee; Merck: Other: Scientific Steering Committee. Castellino:SeaGen Inc.: Other: Scientific Advisory Committee - No honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Committee.